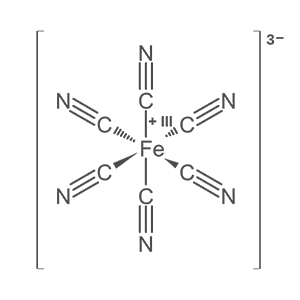
Prussian blue (also known as Berlin blue, Brandenburg blue, Parisian and Paris blue) is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. Turnbull’s blue is essentially identical chemically, excepting that it has different impurities and particle sizes—because it is made from different reagents—and thus it has a slightly different color.
Prussian blue was created in the early 18th century and is the first modern synthetic pigment. It is prepared as a very fine colloidal dispersion, because the compound is not soluble in water. It contains variable amounts of other ions and its appearance depends sensitively on the size of the colloidal particles. The pigment is used in paints, it became prominent in 19th-century aizuri-e Japanese woodblock prints, and it is the traditional ‘blue’ in technical blueprints.
In medicine, orally administered Prussian blue is used as an antidote for certain kinds of heavy metal poisoning, e.g., by thallium(I) and radioactive isotopes of caesium. The therapy exploits Prussian blue’s ion-exchange properties and high affinity for certain ‘soft’ metal cations. It is on the World Health Organization’s List of Essential Medicines, the most important medications needed in a basic health system.
Prussian blue lent its name to prussic acid (hydrogen cyanide) derived from it. In German, hydrogen cyanide is called Blausäure (‘blue acid’). Cyanide also acquired its name from this relationship.
Prussian blue pigment is significant since it was the first stable and relatively lightfast blue pigment to be widely used since the loss of knowledge regarding the synthesis of Egyptian blue. European painters had previously used a number of pigments such as indigo dye, smalt, and Tyrian purple, and the extremely expensive ultramarine made from lapis lazuli. Japanese painters and woodblock print artists, likewise, did not have access to a long-lasting blue pigment until they began to import Prussian blue from Europe.
Prussian blue was probably synthesized for the first time by the paint maker Johann Jacob Diesbach in Berlin around 1706. The pigment is believed to have been accidentally created when Diesbach used potash tainted with blood to create some red cochineal dye. The original dye required potash, ferric sulfate, and dried cochineal insects. Instead, the blood, potash, and iron sulfate reacted to create a compound known as iron ferrocyanide, which, unlike the desired red pigment, has a very distinct blue hue. It was named Preußisch blau and Berlinisch Blau in 1709 by its first trader.
The Daily Omnivore
Everything is Interesting

Leave a comment